In this problem, we are tasked to solve the mass percent of bromine in zinc bromate. First, we need to solve for the molar mass of the substance, then divide the total mass of bromine by the molar mass. It is obtained by dividing the mass of 1 mol of an element (molar mass) by the mass of the compound:
The vapor pressure of diethyl ether (molar mass, 74.12 g mol | Quizlet
The mass percent of bromine in zinc bromate, Zn(BrO₃)₂, is calculated to be approximately 25%, with zinc being the predominant element by mass. Explanation: To calculate the mass percent of bromine in zinc bromate, Zn(BrO₃)₂, we first need to find the molar mass of the compound and the total mass of bromine within it. The molar mass of
Source Image: mdpi.com
Download Image
Percent; Zn (Zinc) 65.409 ÷ 209.3106 = 0.312497: × 100 = 31.2497%: Br (Bromine) … ZnBrO4 Element Mass Percent Bromine 79.904g Bromine 79.904g Zinc 65.409g Zinc 65.409g Oxygen 63.9976g Oxygen 63.9976g ZnBrO4 # of Atoms Oxygen 4 Oxygen 4 Zinc 1 Zinc 1 Bromine 1 … It will calculate the total mass along with the elemental composition and mass

Source Image: numerade.com
Download Image
SOLVED: This is the chemical formula for zinc bromate: Zn(BrO3)2 Calculate the mass percent of bromine in zinc bromate. Round your answer to the nearest percentage.
Identify the characteristics common to all protists. 1 / 4. Find step-by-step Chemistry solutions and your answer to the following textbook question: This is the chemical formula for zinc bromate: Zn (BrO3)₂ Calculate the mass percent of bromine in zinc bromate. Round your answer to the nearest percentage..

Source Image: numerade.com
Download Image
Calculate The Mass Percent Of Bromine In Zinc Bromate
Identify the characteristics common to all protists. 1 / 4. Find step-by-step Chemistry solutions and your answer to the following textbook question: This is the chemical formula for zinc bromate: Zn (BrO3)₂ Calculate the mass percent of bromine in zinc bromate. Round your answer to the nearest percentage..
VIDEO ANSWER: Good day- and this contrast solve for the mass percent of bromine and zinc bromine, so first we need to identify the molar mass of the zinc bromide. … This is the chemical formula for zinc bromate: Zn(BrO3)2 Calculate the mass percent of bromine in zinc bromate. Round your answer to the nearest percentage. Video Answer:
SOLVED: This is the chemical formula for zinc bromate Zn(BrO3)2: Calculate the mass percent of oxygen in zinc bromate. Round your answer to the nearest percentage.
Answer 6 hours ago Solution To calculate the mass percent of an element in a compound, you need to know the molar mass of the compound and the molar mass of the element in question. Step 1: Calculate the molar mass of zinc bromate (Zn (BrO3)2) The molar mass of a compound is calculated by adding up the molar masses of each element in the compound.
Science Bowl Questions – Chemistry, Set 2

Source Image: yumpu.com
Download Image
Zn(BrO3)2 Percent Mass – YouTube
Answer 6 hours ago Solution To calculate the mass percent of an element in a compound, you need to know the molar mass of the compound and the molar mass of the element in question. Step 1: Calculate the molar mass of zinc bromate (Zn (BrO3)2) The molar mass of a compound is calculated by adding up the molar masses of each element in the compound.

Source Image: youtube.com
Download Image
The vapor pressure of diethyl ether (molar mass, 74.12 g mol | Quizlet
In this problem, we are tasked to solve the mass percent of bromine in zinc bromate. First, we need to solve for the molar mass of the substance, then divide the total mass of bromine by the molar mass. It is obtained by dividing the mass of 1 mol of an element (molar mass) by the mass of the compound:

Source Image: quizlet.com
Download Image
SOLVED: This is the chemical formula for zinc bromate: Zn(BrO3)2 Calculate the mass percent of bromine in zinc bromate. Round your answer to the nearest percentage.
Percent; Zn (Zinc) 65.409 ÷ 209.3106 = 0.312497: × 100 = 31.2497%: Br (Bromine) … ZnBrO4 Element Mass Percent Bromine 79.904g Bromine 79.904g Zinc 65.409g Zinc 65.409g Oxygen 63.9976g Oxygen 63.9976g ZnBrO4 # of Atoms Oxygen 4 Oxygen 4 Zinc 1 Zinc 1 Bromine 1 … It will calculate the total mass along with the elemental composition and mass

Source Image: numerade.com
Download Image
Critical Review on Bromate Formation during Ozonation and Control Options for Its Minimization | Environmental Science & Technology
Jan 22, 2023Video Answer Solved by verified expert Created on Jan. 22, 2023, 6:47 p.m. Best Matched Videos Solved By Our Top Educators Transcript VIDEO ANSWER: Here in this problem, we have to calculate the mass percent of bromine in zinc bromide, and this is zinc. Bromide first we’ll find out the molar …

Source Image: pubs.acs.org
Download Image
SOLVED: This is the chemical formula for zinc bromate: ZnBrO₃₂. Calculate the mass percent of bromine in zinc bromate. Round your answer to the nearest percentage.
Identify the characteristics common to all protists. 1 / 4. Find step-by-step Chemistry solutions and your answer to the following textbook question: This is the chemical formula for zinc bromate: Zn (BrO3)₂ Calculate the mass percent of bromine in zinc bromate. Round your answer to the nearest percentage..

Source Image: numerade.com
Download Image
SOLVED: This is the chemical formula for zinc bromate: Zn(BrO₃)₂. Calculate the mass percent of bromine in zinc bromate. Round your answer to the nearest percentage.
VIDEO ANSWER: Good day- and this contrast solve for the mass percent of bromine and zinc bromine, so first we need to identify the molar mass of the zinc bromide. … This is the chemical formula for zinc bromate: Zn(BrO3)2 Calculate the mass percent of bromine in zinc bromate. Round your answer to the nearest percentage. Video Answer:
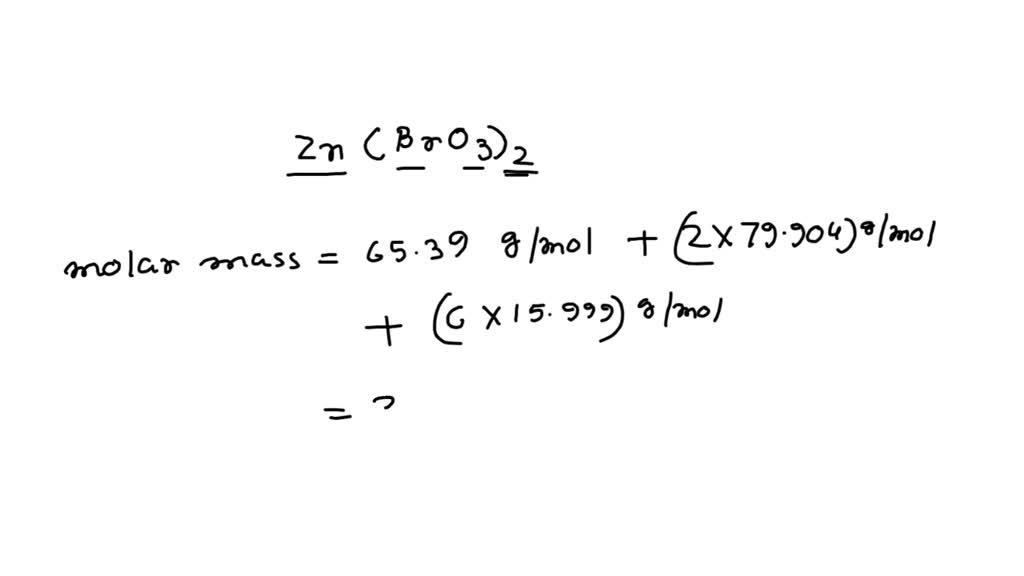
Source Image: numerade.com
Download Image
Zn(BrO3)2 Percent Mass – YouTube
SOLVED: This is the chemical formula for zinc bromate: Zn(BrO₃)₂. Calculate the mass percent of bromine in zinc bromate. Round your answer to the nearest percentage.
The mass percent of bromine in zinc bromate, Zn(BrO₃)₂, is calculated to be approximately 25%, with zinc being the predominant element by mass. Explanation: To calculate the mass percent of bromine in zinc bromate, Zn(BrO₃)₂, we first need to find the molar mass of the compound and the total mass of bromine within it. The molar mass of
SOLVED: This is the chemical formula for zinc bromate: Zn(BrO3)2 Calculate the mass percent of bromine in zinc bromate. Round your answer to the nearest percentage. SOLVED: This is the chemical formula for zinc bromate: ZnBrO₃₂. Calculate the mass percent of bromine in zinc bromate. Round your answer to the nearest percentage.
Jan 22, 2023Video Answer Solved by verified expert Created on Jan. 22, 2023, 6:47 p.m. Best Matched Videos Solved By Our Top Educators Transcript VIDEO ANSWER: Here in this problem, we have to calculate the mass percent of bromine in zinc bromide, and this is zinc. Bromide first we’ll find out the molar …